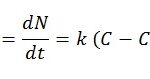Crystallization is a method of separation in which a solid (either crystal or precipitate) is formed from a homogenous, liquid or gaseous phase. The solid formed can be very pure, thus crystallization is also used industrially as a purification process. Salt crystallization, also known as brine treatment, is a very common process in industrial wastewater treatment.
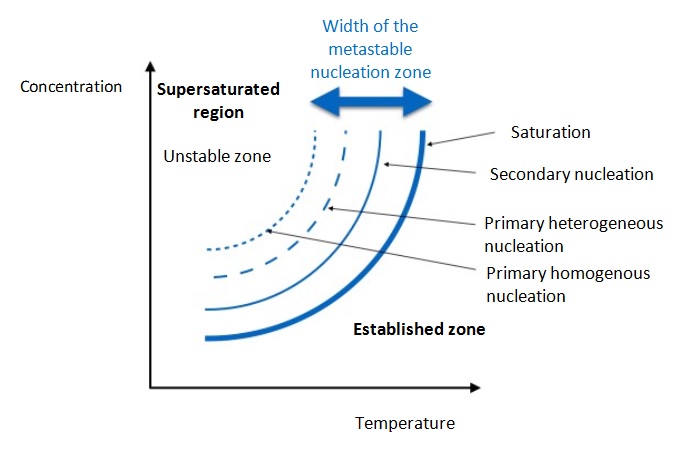
In order for crystallization to occur, it is essential that the solution is supersaturated. Crystallization processes differ from one another with regard to the method for achieving supersaturation. In general, there are three different ways:
- Supersaturation produced by cooling the solution with negligible evaporation.
- Supersaturation produced through solvent evaporation with little cooling.
- Evaporation through a combination of adiabatic evaporation and cooling (vacuum crystallizers).
Note that in order to use crystallizers that achieve supersaturation through cooling, the solutes must have a solubility curve that significantly decreases with the temperature. Where solubility is only slightly temperature dependent, supersaturation is achieved by evaporating the solvent. When a combination of cooling and evaporation is used, a solution is subjected to vacuum conditions to suddenly evaporate the solvent and adiabatically cool the solution. The latter method is the most commonly used industrially to cause supersaturation. In practice, there is a wide variety of industrial crystallizers, each one specifically designed to achieve the optimum form of supersaturation of the solution, depending on its characteristics and properties.
The crystallization process is not simple and the most important stage consists of the formation of solid crystals within the liquid. The solution is concentrated and cooled until the solute concentration is higher than the solubility at this temperature and the solute forms almost pure crystals.
The growth speed of a crystal is known as the crystallization speed. Growth first occurs with the formation of the nucleus, which then gradually grows. When the concentration is higher than the supersaturation, the nucleation, the formation of nuclei, occurs naturally, spontaneously and rapidly. The crystallization speed can be expressed through the following empirical equation:
Where:
- B: nucleation speed (nuclei formed per unit of time and solvent volume)
- N: number of nuclei formed per unit of solvent volume
- t: time
- k, i: empirical parameters
- (C-C*): supersaturation
- C: concentration of solute in the solution
- C*: concentration of the solute saturation
From the equation it can be deduced that the nucleation speed is a direct result of supersaturation. When supersaturation is high, the nucleation speed and crystal growth speed are high, producing small, imperfect crystals with impurities. In contrast, when supersaturation is low, the formation speed is slow and there is regular growth of crystals, producing large, very pure crystals.
Crystallization has numerous industrial applications and obtaining pure crystals is not always the principal objective of this process. It is often the case that the crystallization process is of interest as part of a wider treatment of liquid effluents. In this case, the principal objective is to separate the contamination present in an effluent from the solvent itself in order to obtain the pure solvent and the contamination in solid form, enabling economic management. For example, this application of crystallization is essential in zero waste processes, where the effluent is separated into two streams: one for relatively pure solvent suitable for reuse; and the other for contamination in a solid or semi-solid state.
Thus, crystallization also offers an excellent solution in cases where the principal objective is not obtaining a solid, highly pure product, as in the following applications:
- Treatment of effluents with a high contaminant load
- Treatment of effluents when conventional techniques are not effective (as is the case with brine)
- Impossible to dump treated effluents
- Treatment of effluents with a fluctuating and highly variable composition
Currently, there are crystallizers that are very competitive as regards energy efficiency, based on heat pump vacuum evaporation, and very robust in terms of operation.