Sections
- Concept
- Influential factors
- Main uses of evaporators
- Types of evaporators
- Complementary technologies
Vacuum evaporation concept
Vacuum evaporation is a technique which is characterized by transforming liquid effluent into two flows, one of high quality water and the other comprising a concentrated waste. The water obtained is of sufficiently high quality to be re-used, whereas the waste can be concentrated, even reaching almost total dryness. Waste management costs decrease markedly when concentrating the waste to this extent.
This technique represents a major breakthrough in the treatment of liquid effluents as it allows effluents that cannot viably be treated using physicochemical or biological techniques to be treated in a clean, efficient, safe and compact manner. Vacuum evaporation results in a dramatic reduction in the volume of liquid waste (with the resulting savings in waste management), the concentration of corrosive or scale-producing waste, reuse of the water recovered and the implementation of a zero liquid discharge system, amongst many other advantages.
Evaporation is a unit operation that consists of concentrating a solution by eliminating the solvent by boiling. In this case, it is performed at a pressure lower than atmospheric pressure. Thus, the boiling temperature is much lower than that at atmospheric pressure, thereby resulting in notable energy savings.
The equipment required is compact, practical and instrumentalized, thus meaning that operational monitoring is simple and allowing effluent flows of up to 20 m3/h to be treated in a single evaporator. It should also be noted that as the effluent does not need to be heated to high temperatures, as the water boils at 35-40°C (depending on the operating pressure) when working under vacuum, the evaporator’s energy requirements need not be high quality power supplies and excess energy from other processes will be of use in the majority of cases.
After a process of evaporation, very high percentages of distilled water are achieved (95% at least) and a very low amount of rejection (no more than 5%) to be managed. This rejection is so low due to the high concentration of residues that the process achieves and depending on the composition of the effluent, industrial vacuum evaporators can be appropriate to recover raw materials diluted in the water, which could be sold or re-used.
In summary, evaporation is a novel, efficient and competitive technology that provides very good results as regards treating those effluents that prove complicated to treat using other techniques. This technique often allows the implementation of zero waste policies, with all their inherently positive environmental repercussions. In addition, as a result of the lower quantity of waste generated and the production of a high quality water flow, the initial investment is recovered relatively quickly. Furthermore, this is even faster if excess energy from another process can be used.
| Altitude (m) | Atmospheric pressure (mm Hg) | Boiling (ºC) | |
|---|---|---|---|
| Seal level | 0 | 760 | 100.0 |
| Statue of Liberty | 93 | 750 | 99.7 |
| Empire State building | 381 | 725 | 98.7 |
| Summit of Aneto | 3,404 | 497 | 88.4 |
| Flight in a light aircraft | 5,000 | 398 | 83.0 |
| Summit of Everest | 8,848 | 225 | 68.0 |
| Flight in a commercial aircraft | 12,000 | 150 | 53.5 |
This is the principle working behind vacuum evaporators
Influential factors related to evaporation process
Evaporation is an operation that is controlled by the rate of heat transfer, and the evaporation rate depends on the following factors:
1. Temperature difference between the heating agent and the liquid to be evaporated.
The boiling temperature of the liquid to be evaporated increases as it becomes more concentrated. However, as the process is conducted under vacuum, the temperature difference between the heating agent and the liquid to be evaporated is greater as the boiling temperature if the mixture is much lower than that corresponding to atmospheric pressure. Higher temperature differences lead to higher evaporation rates.
2. Exchange area
The effective exchange area depends on the geometry of the equipment and phenomena inherent to concentration of the solution, such as the deposition of solids or crust formation on the exchange surface. Larger areas lead to a higher heat-exchange capacity and higher evaporation rate.
3. Overall heat-transfer coefficient (U)
This coefficient depends on the physical properties of the fluids concerned (heating agent and liquid to be evaporated), the materials of the walls at which heat exchange occurs, the design and geometry of the equipment, and flow parameters (fluid circulation rates, etc.). Higher values for this coefficient imply a greater ease of heat exchange in the equipment.
4. Properties of the liquid to be evaporated
The viscosity, possibility of foam formation, ability to corrode, etc. all have practical effects on the rate of heat transfer.
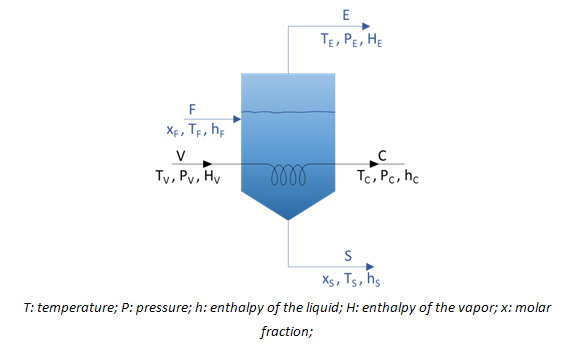
The key parameter when designing the evaporator is the exchange area required for evaporation. Both mass and energy balances must be considered when calculating this area. Thus, for an evaporator into which a flow F is fed and two flows (the concentrate S and distillate E) are removed, as shown in the figure:
The following mass balances must be considered:
Overall mass balance:
F = E + S
V = C
Mass balance for the solute:
F xF = S xS
And the following energy balances:
V HV + F hF = C hC + E HE + S hS
Q = V HV – C hC = V (HV – hC) = U A >T
where Q is the heat flow transmitted via the heating surface of the evaporator, U the overall heat-transfer coefficient, A the area required for evaporation, and >T the temperature difference between the heating agent and the liquid to be evaporated.
Main Uses Of Vacuum Evaporators
Another factor to mention regarding vacuum evaporators is their versatility, and the large number of situations in which they can be applied (providing that the results justify the necessary investment for their installation, as it isn’t the cheapest technology).
Thanks to this, industries that have to treat medium or large flows can benefit from important savings, as the volume of residues that they have to send to be managed is considerably reduced. This technology is also very suitable for the production of high quality water that numerous industries require for incorporation in their productive processes.
Vacuum evaporators are a competitive and efficient solution for treating wastewater for which acceptable results cannot be achieved using more conventional methods (physicochemical and biological treatments). This typically occurs when the effluent contains:
- very high concentration of salts,/li>
- non-biodegradable compounds,
- substances that are toxic to microorganisms
- metals
- etc.
-
Such effluents are produced industrially by general services:
- boiler purging,
- ion exchange resin regeneration effluents,
- reverse osmosis rejection processes,
- process water treatment sludges,
- cooling tower purges, etc.,
as well as specific effluents from:
- the food industry (brine treatments),
- the electroplating industry (depleted baths, wash and surface treatment waters),
- the chemical, pharmaceutical and cosmetics industries (tank and reactor washing waters, etc.),
- the paint manufacturing industry (reactor washing),
- the car and metal industries (oil-based emulsions, degreasers, cutting fluids, penetrating fluids),
- the graphical arts industry (ink treatment and concentration and roller washing waters),
- waste management companies (landfill leachates, high conductivity waters, etc.),
- hospital waste, etc.
- In addition to its use during effluent treatment, evaporation is also widely used in the food industry to concentrate many types of heat-sensitive substances (to concentrate fruit juices, produce condensed milk, remove alcohol to obtain alcohol-free beer, etc.).
Types Of Vacuum Evaporators
One of the factors that results in important operational difference between different vacuum evaporators is the type of technology used to heat the effluent to be evaporated, an aspect that also affects operating costs. Thus, we can find:
A. Heat pump vacuum evaporators
Operation of this system is based on the refrigeration cycle of gas contained in a closed loop. The refrigeration gas is compressed by a compressor, as a result of which its temperature and pressure increase. It then circulates through the heat exchanger of the evaporator itself, heating the feed. As the system operates under vacuum, the boiling temperature is around 40 ºC. The refrigeration liquid leaves the evaporator’s exchanger and is decompressed and cooled using an expansion valve. Passage through a second heat exchanger (the condenser) causes the vapor formed in the evaporator to condense and its temperature to increase immediately prior to passing through the compressor again, thus repeating the cycle. The same refrigeration fluid allows the feed to be evaporated and the vapor generated to be condensed, therefore the system does not require any other heating or refrigeration source. This means that the process is highly advantageous from an economic and management viewpoint.
It is an ideal technology for treating not particularly high flows of corrosive, scale-producing, or viscous liquids. Its operation typically requires an energy consumption of 130-170 kWh per cubic meter distillate.
B. Mechanical vapor recompression vacuum evaporators
This technology is based on recovery of the heat of condensation of the distillate as a heat source for evaporating the feed. To this end, the temperature of the vapor generated upon evaporation is increased by mechanical compression. Upon passing through the exchanger of the evaporator itself, this compressed, and therefore superheated, vapor has two effects: (1) it heats the liquid to be evaporated and (2) it condenses, thereby reducing the need for a refrigeration fluid.
It is a very efficient and competitive evaporation system, with an energy consumption of around 50-60 kWh per cubic meter of distillate obtained.
C. Multiple effect vacuum evaporators (MED)
This technology comprises a series of mutually connected evaporators in which the vacuum steadily increases from first to last. This means that, in principle, the boiling temperature decreases, thus allowing the vapor generated in an evaporator (or effect) to be used as heating fluid in the following effect.
Its main advantage with respect to a single evaporator is the saving in both heating fluid and refrigeration fluid. This is one of the economically most competitive options for treating high flows. In these tanks, the water is distributed in thin films in order to facilitate evaporation to reduce pressure.
The phenomenon of progressive pressure reduction allows the feed water to continually undergo both liquefaction and evaporation processes without the need for a heating system.
These processes operate at temperatures around 70°.
D. Multi-stage flash distillation (MSF)
Multistage flash evaporation is widely used in the industrial sector and involves heating the feed liquid in a vessel and immediately driving water through a system of heating pipes in which part of the water is vaporized. It then passes to another vessel in which temperature and pressure are such that a portion of hot water is suddenly vaporized, leaving a concentrated remnant in liquid form that is passed on to fuel the next stage.
Afterwards, the vapor is allowed to cool until it liquefies and is then collected, free of impurities. This process is then repeated at another stage. After a determined series of stages, we achieve water that has been distilled repeatedly in a very quick manner, therefore containing a low quantity of dissolved contaminants.
This type of evaporation operates at temperatures between 90° and 120°.
In summary, vacuum evaporation allows the treatment of flows which, as a result of their composition, characteristics, or management complexity, cannot be treated using conventional physicochemical techniques. In addition, with a reduced energy consumption, this technique allows the volume of waste generated to be significantly reduced, a significant flow of water to be recovered for reuse, and the implementation of a zero-waste system with readily assumable economic cost.
Although these systems are simple to operate, it is essential that the choice and design of the most suitable equipment for specific needs is performed by a team of experts in this technology.
Complementary Technologies For Vacuum Evaporation
It is usual for a process of vacuum evaporation for wastewater treatment to be completed with other technologies, then, vacuum evaporators can work as a unique solution or integrated in a bigger wastewater treatment plant. These complementary effluent treatment technologies can be applied:
1) Pre-treatment technologies, such as lamella separators, DAF units, chemicals dosing, or even membranes technologies.
2) Post-treatment technologies: When we need to obtain a high concentration of waste or zero
liquid discharge is required, the most appropriate technology is crystallizers, which can be used in two ways:
- operate with an evaporator-crystallizer
- add a crystallization stage after the vacuum evaporator
The composition of the effluent and the flow rates are the key to decide what is the best option. The combination of the two technologies is especially suitable for heavily polluted waters, brines and emulsions. This is what happens with the oil of oily water, which can be sold as a secondary product with a water content of less than 5%, or with the recuperation of aluminum hydroxide, which can subsequently be used as a chemical product, to mention just a few examples.
After the crystallization we can obtain around 99% of ultrapure water and a highly concentrated rejection of salts, oils, etc.
Industrial evaporators and crystallizers are thought to be more expensive than other technologies due to their high energy consumption, but the gap is getting closer thanks to the improvements made in the last years. On the other hand, when cogeneration is possible (and it is frequently) the cost of feeding the evaporator is removed.
Moreover, the big savings that companies obtain in waste management during the evaporator’s life have to be considered. The amount of concentrated waste that companies get after the evaporation process is so small that the expenses in waste management tend to zero.
Last, but not least, it’s important to point that they can operate automatically and the maintenance is very simple.
Obviously, vacuum evaporation and crystallization are not the best option for every case, but they are always worth to be considered in industries that need to treat their wastewater.
Some advantages
- High quality water.
- Up to 99% treatment efficiency (using Vacumm Evaporation + Crystallization).
- Enables the reuse of treated water.
- Can treat more complex effluents
- Low power consumption
- Zero liquid discharge.
- Flexible and compact design, easy maintenance.
- No external heat sources.
- Low waste disposal.